Synthetic materials
Rayon, synthetic leather and foam: How synthetic materials replaced - and became superior to - natural materials
Nature offers many materials with special properties, such as leather, glass, wool, metal, wood, which are utilised in every-day life, mostly in a form that is tailored to the specific application.
This is also true for football: for many years balls and shoes were made of leather, clothing was usually made of cotton, whistles were made of metal, the goal posts of wood and the game was played on grass. Today, all these natural materials were either completely replaced by synthetic materials or plastic-based alternatives have been developed that are used under certain conditions, for example, artificial turf, which requires far less maintenance than natural grass.
In the early 20th century, some synthetic materials had been specifically developed as alternatives for certain natural products made of raw materials that were difficult to obtain in large quantities, for example, because they came from far-away countries or were of animal origin. A common material at the turn of the century was galalith, a type of synthetic horn made of casein and formaldehyde, which was used as a low-cost substitute for horn of animal origin or ivory, for example, to produce buttons. Another popular example of that time is synthetic rubber.
From a chemical perspective most plastics are so-called "organic polymers" made up of molecules consisting of repeating units of one or several different basic components (called "monomers" in technical jargon). A polymer molecule typically consists of between one thousand and one million of such monomers, bound to each other in very long chains (cf. Figure 1). A finished plastic product again consists of millions of such intertwined or cross-linked polymer chains.
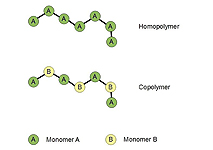
![]() Figure 1: Polymer molecules are made up of many components, the monomers, linked to each other in chains like a pearl necklace.
Figure 1: Polymer molecules are made up of many components, the monomers, linked to each other in chains like a pearl necklace.
The chemical structure of "monomers" determines the fundamental chemical and physical properties of the plastic material. Owing to its chemical structure, it is either, for example, water-soluble or water-repellent, scratch-proof or has fibre-forming properties. The arrangement of the polymer chains (consisting of monomers) in the finished product also determines the properties of the material, for example, workability, elasticity or breaking strength.
The technical properties of plastics can be modified or adjusted within wide limits by the choice of monomers and the manufacturing or processing process or also by using additives (such as pigments or softening agents). By this means the properties of natural materials can be copied or even specifically improved and adjusted to meet special requirements.
Polyamides in particular are a many-purpose and cost-effective class of plastics.
Depending on the base material, there are two different types of polyamides: the so-called "Perlon type" formed by amino carbon acids and the "nylon type" formed by dicarbon acids and diamines (cf. Figure 2).
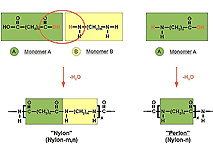
![]() Figure 2: Polyamides are produced in several steps, based on one monomer: amino carbon acid ("Perlon" or "nylon-n"), or based on two monomers: dicarbon acid and diamine ("nylon" or "nylon-m,n")
Figure 2: Polyamides are produced in several steps, based on one monomer: amino carbon acid ("Perlon" or "nylon-n"), or based on two monomers: dicarbon acid and diamine ("nylon" or "nylon-m,n")
In the mid-1930s, a research team of the US company DuPont, headed by Wallace H. Carothers, developed the first nylon fibres in the USA (US 2,130,948) as a synthetic substitute for natural silk. In Germany, I.G.-Farbenindustrie AG introduced polyamides with a similar structure, Perlon (meanwhile also called nylon), which did not infringe the patent on nylon. Within few years, polyamide became the favourite material for producing stockings, thanks to its silky quality, its fineness and its crystalline transparency.
Most of the polyamides used for technical applications possess high strength, stiffness and tenacity, and high chemical resistance and processability. These properties are based to a large extent on the amide groups of polymer chains interacting through hydrogen bonds, thus facilitating the formation of large crystalline regions and leading to a relatively stable structure (cf. Figure 3). This crystallinity is responsible for the polyamides' tendency to form fibres. Polyamides are also very important as thermoplastic materials.
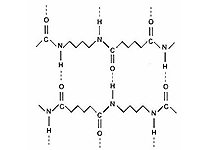
![]() Figure 3: The formation of highly crystalline regions due to hydrogen bonding between neighbouring amide groups is responsible for the high strength of the material and its capacity of fibre-forming
Figure 3: The formation of highly crystalline regions due to hydrogen bonding between neighbouring amide groups is responsible for the high strength of the material and its capacity of fibre-forming
Non-fibrous polyamide has high wear resistance and good sliding properties. Glass or carbon fibres are added to further improve the mechanical properties. Its miscibility with polyethylene and polyurethanes also allows to vary the viscoelastic properties. Elasticity, fracture strength and other properties of polyamide-based materials can be widely modified and tailored to meet the special requirements in practice.
Apart from nylon stockings we encounter fibrous polyamide as fishing lines, trimmer lines for garden strimmers, strings for tennis rackets, wall-to-wall carpeting, toothbrushes or ropes of all sizes (for example climbing ropes). Non-fibrous polyamide is used to produce unbreakable household articles or technical components that are highly resistant to abrasion, such as slide bearings, gears, rollers as well as plugs or screws.
The largest portion of polyamide production is used for synthetic fibres for textiles (sportswear among others). The raw material is white and can be dyed before processing, for example, with disperse dye or direct dyestuff, today frequently also with reactive dyes.
Strength, tenacity and abrasion resistance are also the essential material properties required for the soles of football boots. Another important property of polyamide is that it is light-weight - like virtually all plastics - compared to many other materials, for example, leather or metal. Consequently, it is an ideal material for the soles of football boots and has been used for this purpose from the late 1950s until today. The development of serviceable shoes with polyamide soles took place between the World Cups of 1954 and 1958. Figure 4 shows the boots of Just Fontaine who scored 13 goals in the tournament in Sweden, an unmatched record until today.

![]() Figure 4a: Only once, in 1958, a single player scored 13 goals in one World Cup tournament. Due to the stress and strain imposed on the material during the tournament, the boots with which Just Fontaine scored the record had already developed some cracks in the light-weight but still not very flexible nylon soles.
Figure 4a: Only once, in 1958, a single player scored 13 goals in one World Cup tournament. Due to the stress and strain imposed on the material during the tournament, the boots with which Just Fontaine scored the record had already developed some cracks in the light-weight but still not very flexible nylon soles.
In the course of time, to improve the bending flexibility of the sole the originally used pure nylon-6,6 was replaced by more flexible plastics or the latter were added to the polyamide, for example, polyurethane. Furthermore, modern soles of shoes often also have reinforcing elements and resilient moulded bodies to enhance stability and to confer springy-elastic properties to the sole (Figure 5; for more details see the chapter "Football Footwear", section 3.4.).

![]() Figure 5: Football boot with springy-elastic polyamide sole with reinforcing elements and resilient moulded bodies (approx. 1978)
Figure 5: Football boot with springy-elastic polyamide sole with reinforcing elements and resilient moulded bodies (approx. 1978)
Figure 5: Football boot with springy-elastic polyamide sole with reinforcing elements and resilient moulded bodies (approx. 1978)
Due to the above mentioned properties, polyamide (often mixed with other synthetic materials) is the most common class of plastics used in football applications. Cast polyamide is used for outsoles, but polyamide in various different forms fulfils several other functions of the shoe: fibrous polyamide is used for the blended fabric of inlay soles while polyamide film is used for the stretch-resistant coating of the toe caps or shoe tongue. The stability of polyamide fibres is also utilised in tear-proof jerseys or abrasion-resistant blades of artificial grass.
It is likely to assume that Carothers and his research team had industrial or everyday applications in mind when developing nylon fibres. However, they also essentially contributed to making football faster and more athletic. Particularly in the regions of northern or central Europe the speed of modern football would not have been conceivable without the introduction of light-weight plastics, because rain-soaked jerseys, boots or footballs, made of natural materials such as wool or leather, often weigh several times their original weight.
The following table shows a compilation of frequently used classes of plastics. A material, other than polyamide, that is currently very common in football applications is polyurethane. It has replaced PVC as surface material in synthetic leather. Foamed polyurethane is used as cushioning and shock-absorbing material. Other materials used are, above all, polyethylene, polypropylene, polyester and EVA, often blended with polyamide.
| Class of polymers | special properties | forms, applications | examples from the wider field of football |
|---|---|---|---|
| Polyamide | crystallinity; fibre-forming; high abrasion resistance, stiffness and tenacity; good sliding properties and high chemical resistance | fibres with high tear strength; injection moulding parts with high stiffness, blended fabrics, e.g. with polyethylene and polyurethanes | outsole of the football boot (DE 1 776 337 U1), component of blended fabrics of inlay soles (WO 02/00051 A1), stretch-resistant coating and seams of shoe tongues (DE 18 864 404 U1), blended fabric of football casings (EP 0598 542, DE 43 39 677); artificial turf (DE 1578824) |
| Polyurethane | foaming by adding water during production, mechanical properties depend on choice of monomers, particularly of the polyol component; special feature: | foamed plastics and cushioning materials in dishwashing sponges, mattresses, shoe soles; varnishes, adhesives, sealants; synthetic leather | bending flexible outsoles of football boots (DE 2847152 C2), springy-elastic midsoles or inlay soles (WO 02/00051 A1), substitute for cotton fibres in protective padding and protective fabrics (DE 27 11 579 A1), shin guards (DE 76 38 546 U1), sticky coating of goalkeeper gloves (DE 195 30 282 A1), modern synthetic leather balls (DE 102 55 092 A1) |
| Polyester | large family of plastics including polycarbonate (PC) and thermoplastic polyethylene terephthalate (PET) among others; broad variety of properties | fibres and micro fibres for textiles and non-woven fabrics; PET bottles; films; extremely tear-proof materials; fibre composites; polyester resins as varnishes | highly elastic player's clothing (DE 698 19 170 T2), blended fabric of football casings (EP 0 598 542 A2, DE 43 39 677 A1); polyterephthalates as moisture-controlling multi-filament yarn for artificial turf (DE 10 2004 013 749 A1) |
| Polyethylene, polypropylene | semi-crystalline, waxy; high chemical resistance; very low water absorption or water vapour permeability; good sliding property; low density; softens at approximately 80 degrees C | films; blow-moulded hollow bodies; vessels and containers; additive to fibres, fabrics or laminates | component of plastic-fibre blended fabrics in inlay soles (WO 02/00051 A1), foams for protective padding for elbows, knees or shins (DE 83 23 979 U1) |
| Polyvinyl chloride | amorphous; thermoplastic; hard and brittle; when softeners and stabilisers are added it becomes soft and pliable | flooring, pipes, wire insulation and cable sheathing, audio records, synthetic leather | early synthetic leather balls (GB 733,154 A), outer shells of shin guards (DE 1899803 U) |
| Phenol formaldehyde resins | hard, very break-proof materials, black to red colouring | synthetic cast resins; binder for hardboards and chipboards; grinding discs; brake linings | early material (approx. 1945) used as substitute for metal in whistles (GB 620,720 A) |
| Polyethylene vinyl acetate (EVA) | copolymer of ethylene and vinyl acetate; rubber-like properties increase with rising proportion of vinyl acetate; high heat resistance and high ageing resistance; | high-quality films (improved elongation at break compared to pure polyethylene); shower curtains; hot melt adhesives; shoe soles | shock-absorbing material in shin guards (DD 148 441 A3), insoles of shoes (DE 10 2004 011 680 A1) or ball casings (DE 102 55 092 A1, US 2004/0213984 A1) |
| Polytetrafluoroethylene (PTFE) | semi-crystalline; very inert; extremely resistant to bases and solvents; low friction coefficient; low wettability | non-stick coating for pans, weather-proof and UV-resistant coatings (e.g. in textiles (Gore-Tex) or glasses) and membranes, dry-film lubricant (analogously: "feet" for computer mice), seals | moisture-vapour permeable and weather-resistant sportswear and undergarment (US 5,155,867 A), stain resistant coating of the insole (DE 10 2004 011 680 A1) |

